Pfizer/BioNTech C4591001 Trial - Data Altered Between EUA & BLA Submissions
A brief look at the latest EUA release, resulting in a few additional evidence of blatant data manipulation.
By way of introduction for new subscribers: the Pfizer-BioNTech C4591001 trial underpinned authorization of the Pfizer-BioNTech COVID-19 vaccine - first under Emergency Use Authorization (EUA) and later via a Biologics License Application (BLA).
This trial’s results were released 6 days after the U.S. election on November 9, 2020 - as Pfizer went to such lengths as to delay the efficacy announcement (despite having met the required endpoints weeks before1, contrary to what they later claimed in a Science.org ‘debunk’2) - thereby denying President Trump a political victory over his then-rival, Joe Biden.
On the basis of the exceptional efficacy initially reported - 95%3, then 90%4 - policymakers went on to adopt (abhorrent) measures such as vaccine mandates, which were encouraged by Pfizer’s lobbying5.
Very common adverse events (AEs) such as menorrhagia went completely undetected, and the reported efficacy was not borne out in real-world data6. Understanding why - and if regulations were infringed, how - is critical to preventing a recurrence.
On June 30, 2025, the Informed Consent Action Network (ICAN) released newly obtained data7: the dataset submitted at the time of the EUA, in December 2020.
This dataset is valuable - and one I requested some time ago - because it offers a different perspective and cutoff date from the BLA submission of March 13, 2021, which has, until now, been the main focus of public scrutiny.
Despite my best efforts, this article is lengthy. For readers who don’t wish to go through the entire piece, here is a short recap of the main anomalies in the data between the EUA and BLA submissions:
Adverse-event onset dates shifted from “very close to vaccination” to “much later.”
358 adverse events removed between versions.
PCR results flipped (NEG → POS).
13,413 changes to V01DT.
43,081 changes to V02DT.”
Several colleagues and I have already covered - at length on this Substack - data-driven findings from last year’s releases, indicating the trial warranted an in-depth, transparent audit at the time of the EUA (December 2020) and BLA (March 13, 2021).
For readers of those two articles, the evidence should be more than sufficient to warrant a thorough investigation of every entity involved in the trial - and to show that genuine informed consent by the public was, by definition, unattainable. In particular, the product ultimately released by Pfizer-BioNTech - except for 252 trial participants - was not the same as the product evaluated in the trial, and the released product was demonstrably more hazardous than the tested product.
With the appointment of Retsef Levi - co-author with
of the original ‘Process 2’ concerns8 - as chair of the CDC’s new COVID-19 Vaccine Working Group, where he will serve alongside , I’m optimistic that transparency and scientific rigor will, at last, prevail.Accordingly - and given that my time is better spent investigating the actors muddying the medical-freedom space than endlessly cataloging new anomalies in Pfizer’s datasets - I won’t devote months to this new tranche of EUA documents.
Still, a brief review of this submission is worthwhile, because it highlights several critical flaws.
Method
The references to all the documents used are provided, as well as the R9 code required to reproduce every figures & charts advanced.
When sources are required, they are provided in footnotes.
EUA Data Released
The dataset comprises two ZIP archives prefixed pd-eua-production-*, hosted on PHMPT.org10.
Assuming you have already downloaded and extracted the BLA production12, we use two R scripts: one to download the EUA data (R13) and a second to extract the ZIP archives and generate a file-inventory summary (R14).
Our primary objective is to verify that no key data fields were altered between the EUA and BLA submissions. As with prior integrity checks on this dataset, the review surfaced several notable discrepancies.
We began with ADSL (subject-level data), which is straightforward to compare because it maps one-to-one to participants. But because the most consequential discrepancies appear in the adverse-event (AE) tables, we summarize those first and return to ADSL later.
EUA vs BLA - Adverse Events Data
We compared two AE files (R15):
- eua_data/xpt_data/FDA-CBER-2021-5683-1231982-1232400_27034_S1_M5_c4591001-ia efficacy-S-ae.xpt
- xpt_data/FDA-CBER-2021-5683-0775805-0776791_125742_S1_M5_C4591001-S-D_ae.xpt.
After verifying that vaccination dates had not changed, we merged BLA ADSL (xpt_data/FDA-CBER-2021-5683-0772469-0773670_125742_S1_M5_C4591001-A-D_adsl.xpt) to add subject-level fields.
In a first pass, we checked whether adverse events present in both datasets - matched via their unique AESPID - showed different dates between the EUA and BLA cuts.
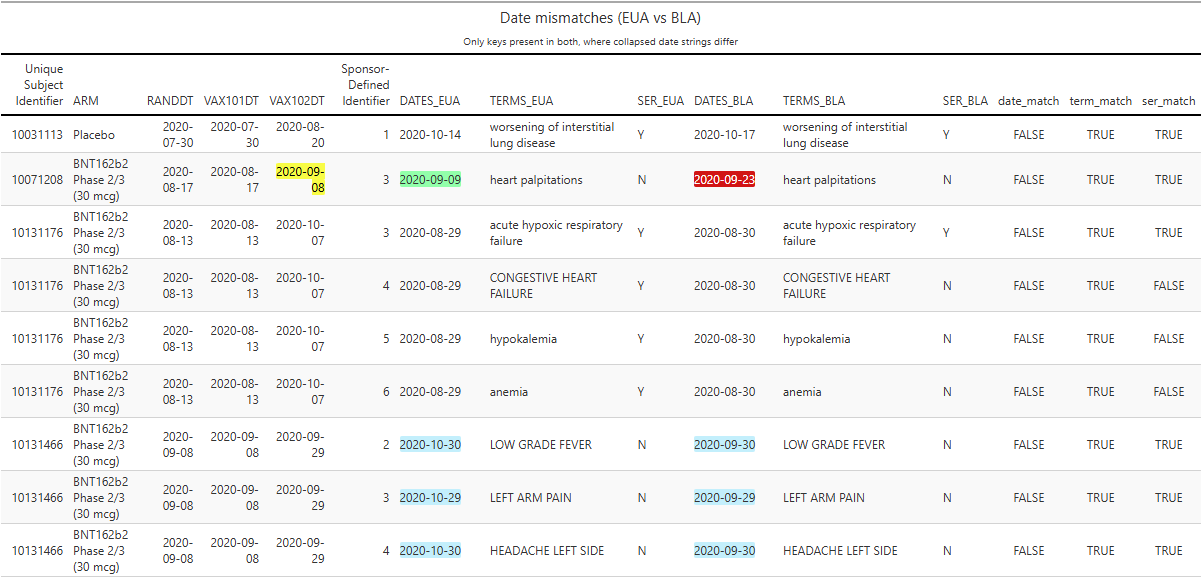
Readers who wish to go deeper can consult the companion full HTML report; even a brief scan reveals troubling patterns. For example:
On 17 Aug 2020, participant 10071208 was randomized to the BNT162b2 arm.
On the same day, they received Dose 1 of BNT162b2.
On 08 Sep 2020, they received Dose 2.
At EUA time, an AE of “heart palpitations” was recorded on 09 Sep 2020 (+1 day post-Dose 2).
At BLA time, the “heart palpitations” AE date had shifted to 23 Sep 2020 (+15 days post-Dose 2).
There are numerous other AE date shifts of similar magnitude - including many moved by roughly one month - as illustrated (highlight in blue) in the excerpted table below.
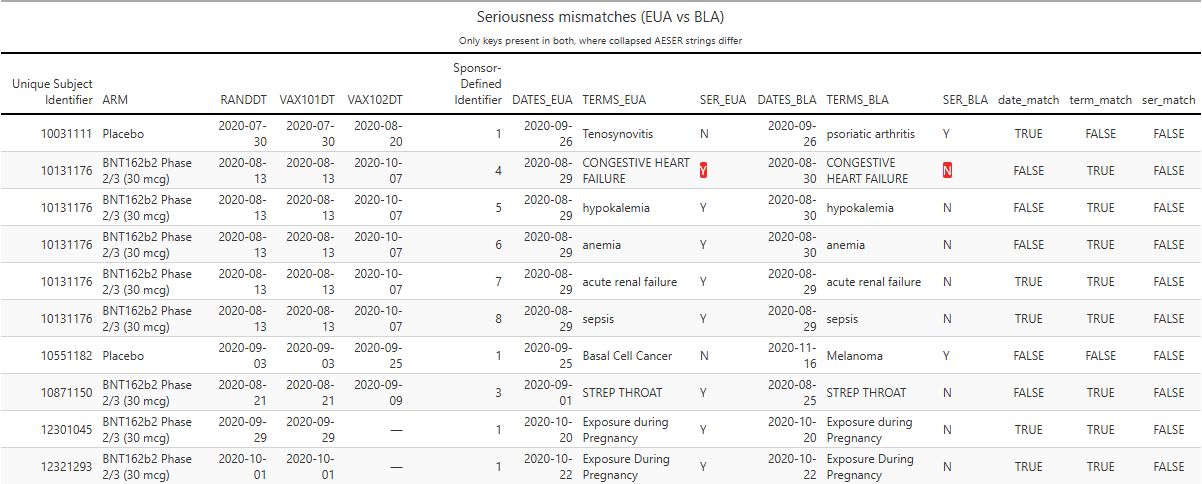
There are also mismatches in the seriousness classification of certain events. For example, participant 10131176’s congestive heart failure was reclassified from serious at EUA time to non-serious at BLA time (see red highlight below).
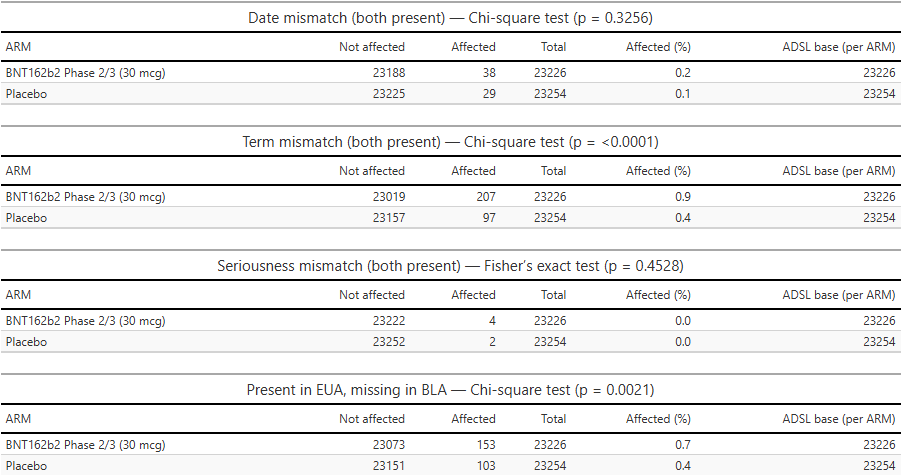
Of the 10 observed reclassifications, 8 were from “serious” to “non-serious”, and 8/10 occurred in BNT162b2 recipients. That distribution is unlikely by chance.
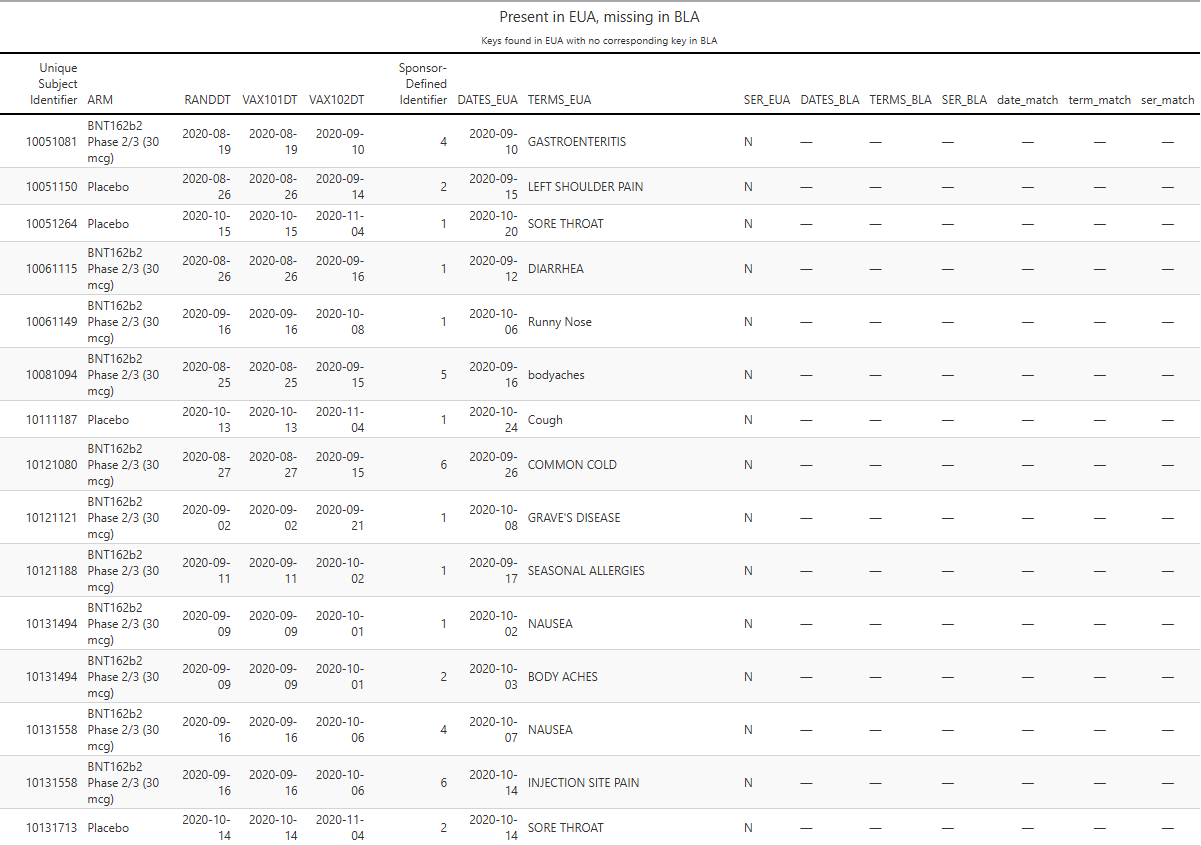
Additionally, 358 adverse events present at EUA time are absent from the BLA dataset.
Some of these could have been re-keyed under a different AESPID - easy to check (R16).
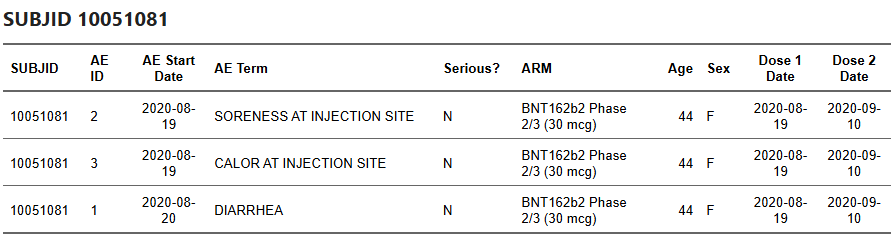
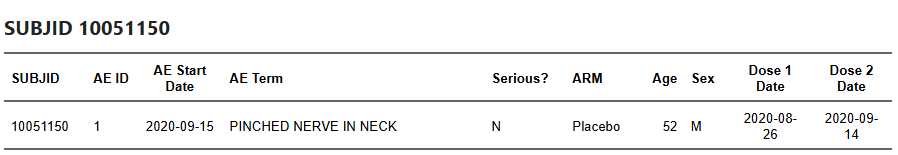
Example: participant 10051081 had Gastroenteritis recorded on 2020-09-10 at EUA time; no corresponding entry remains in the BLA cut.
Participant 10051150 reported Left shoulder pain on 2020-09-15, a day after their second dose on 2020-09-14. In the BLA cut, this record appears as ‘Pinched nerve in neck’.
Participant 10061115 had Diarrhea recorded on 2020-09-12 in the EUA cut; in the BLA cut, the participant has no AE record anymore.
I could enumerate all 358 such cases, but the more constructive step is an independent audit. It demonstrates, in any case, that the adverse events deletion, described by Dr. Augusto Roux17 & Brook Jackson18, were far from rare occurrences. The full list, with keys and comparison logic, is available for regulators and external reviewers to verify.
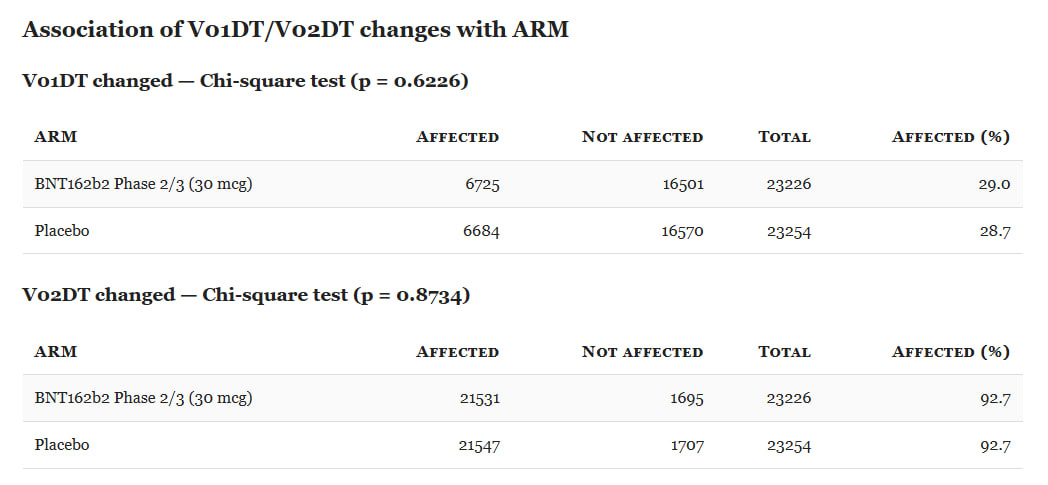
We tested whether these anomalies were arm-specific by comparing all randomized subjects in ADSL with ARM = ‘Placebo’ or ARM = ‘BNT162b2 Phase 2/3 (30 µg)’. A subject was coded Affected if they had ≥1 AE meeting the anomaly definition; otherwise Not affected. We then compared the proportion Affected between arms.
In a well-run trial, such anomalies should be rare (legitimate exceptions include term refinements after diagnostic clarification). In our data, several anomaly types are over-represented in the BNT arm, at levels unlikely to reflect chance alone.
EUA vs BLA - ADSL Files
We compared two ADSL files (R19) :
eua_data/xpt_data/FDA-CBER-2021-5683-1226624-1227706_27034_S1_M5_c4591001-ia efficacy-A-adsl.xptxpt_data/FDA-CBER-2021-5683-0772469-0773670_125742_S1_M5_C4591001-A-D_adsl.xpt
A full HTML report is available. As expected, most changes between the November 2020 (EUA) and March 13, 2021 (BLA) cuts occur in variables that naturally evolve over time (for example, date of last exposure to treatment).
Treatment-arm assignment recorded in ADSL (ARM) is essentially unchanged across cuts. This does not support the hypothesis advanced by
As I argued previously21, a more plausible alternative to the phenomenon they observed is that some records were removed22.
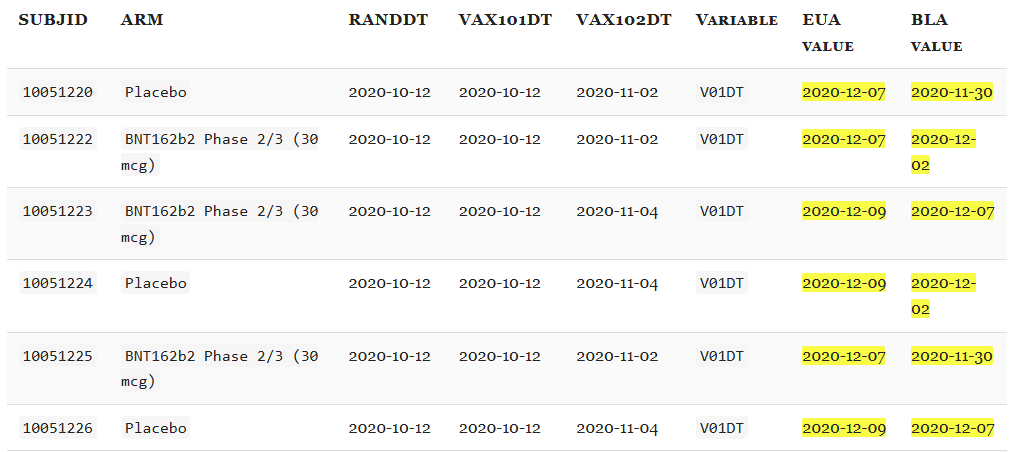
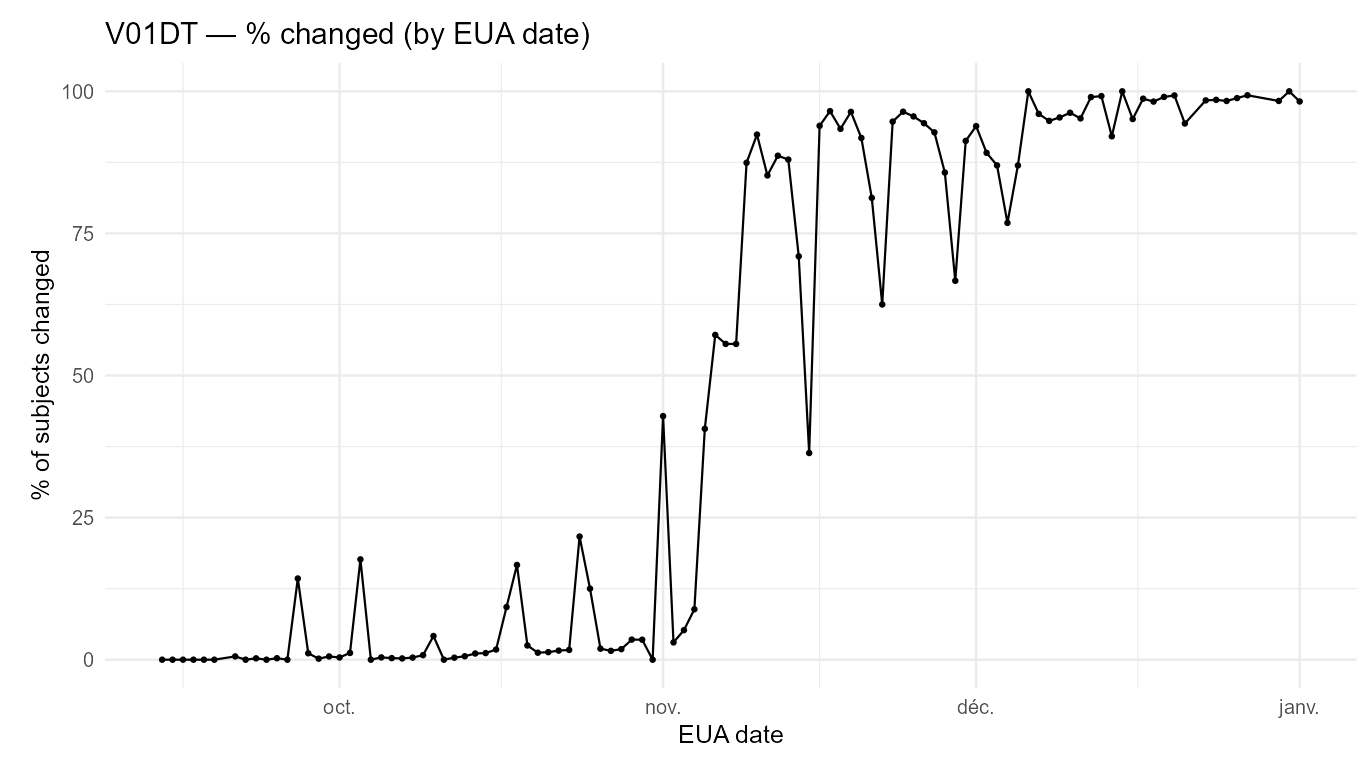
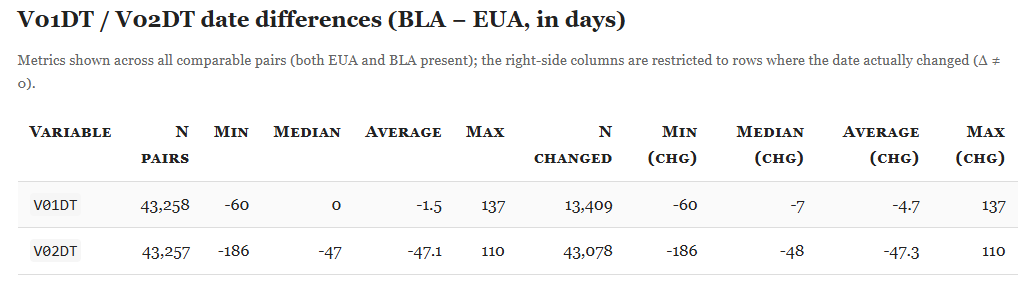
However, several fields that should be invariant changed materially. For example, V01DT (Visit 7 - Date of unblinding or visit at 1 month post dose 2) changed in 13,413 cases.
Some fields, such as V02DT (Visit 8 - Date of unblinding or visit at 6 months post dose 2), changed in 43,081 cases - possibly reflecting a later unblinded analysis. That explanation does not apply to V01DT, nor does it explain why V01DT values shifted between the December 2020 (EUA) and March 13, 2021 (BLA) cuts.
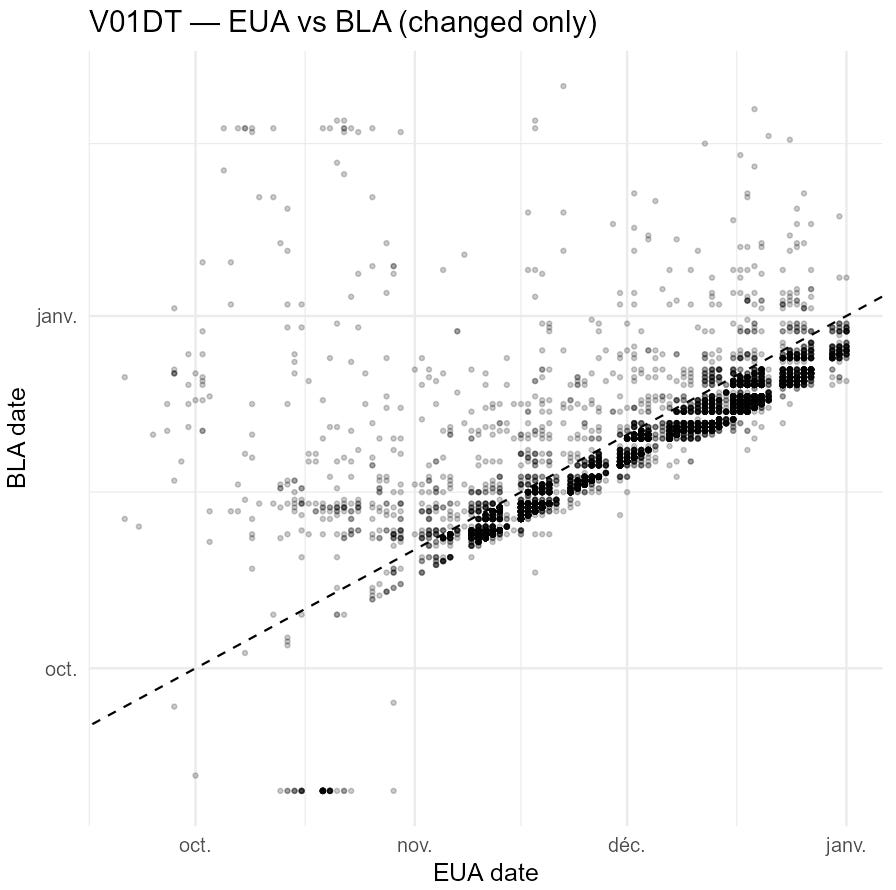
The excerpt below illustrates the nature of the edits: for many participants, the Visit-7 date was moved earlier by ~1 week between the EUA and BLA datasets.
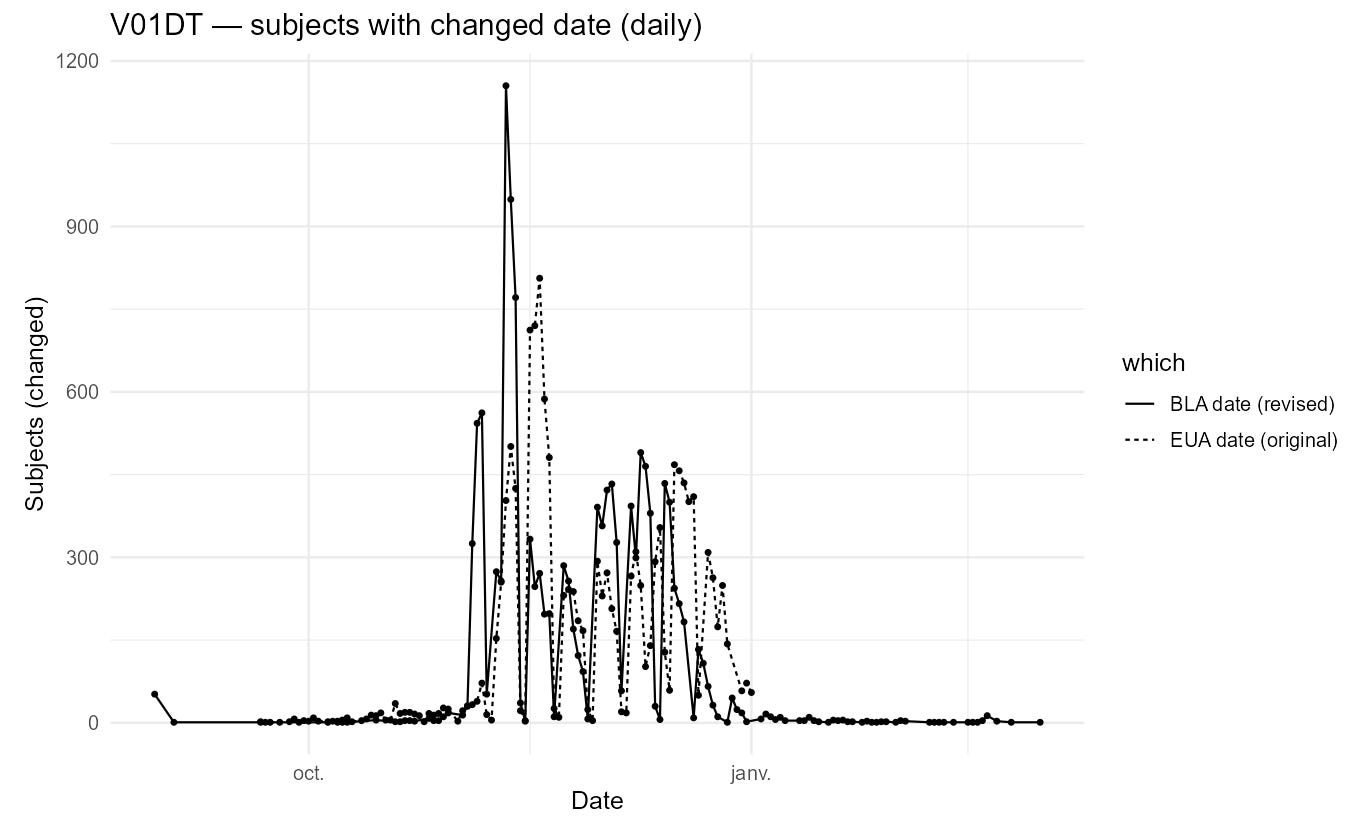
The next plot shows how these edits reshape the apparent visit schedule.
A second plot indicates that the edits are concentrated among participants completing visits later in the trial, with some early visits affected as well.
Finally, the histogram below shows the distribution of day-level shifts (BLA − EUA); positive values indicate that the BLA date is later.
For V02DT, the dates shift earlier by ~1.5 months on average, with a markedly different pattern from V01DT. The hypothesis of a switch in the unblinding anchor (note: UNBLNDDT is absent from the EUA ADSL) does not fully account for this: the change does not affect visits in February 2021, and clear exceptions remain.
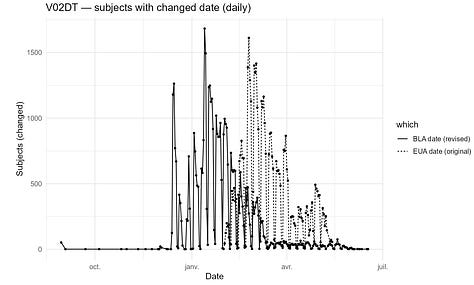
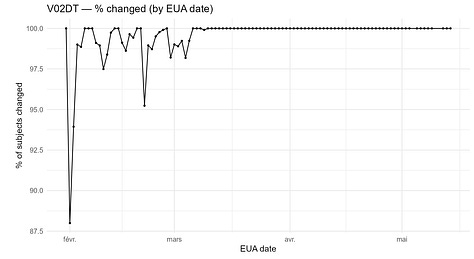
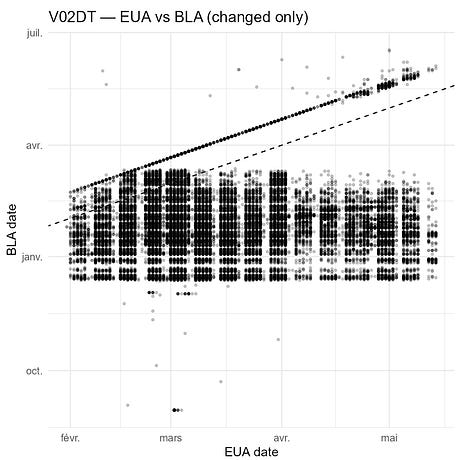
The distribution plot shows irregular shift patterns for both variables.
Finally, there are no statistically significant differences between arms in the share of subjects affected by these V01DT/V02DT changes.
This leaves an obvious question:
What process would create such conspicuous edits?
One plausible motive is analytical: shifting V01DT/V02DT can alter adverse-event analyses. The Analysis Data Reviewer’s Guide (ADRG)23 underscores the role of these visit/unblinding dates in defining risk windows, censoring, and analysis populations.
Another, more operational explanation is that a required non-missing field may have been enforced at EUA time. In that scenario, some visit dates were placeholdered - forward - or back-filled around the EUA approval date (2020-12-11) - and later overwritten with actual values. The apparent clustering of EUA-cut dates near 2020-12-11 would be consistent with such placeholder entries.
Either way, this would be very far from Good Clinical Practice (GCP) and data-integrity principles. I’ll defer to the analyst community and further investigations to pin down the exact motive.
EUA vs BLA - Testing Data Files
For this pass, I examined whether modified dates or test results affected the testing datasets. Reviewing the MB (Microbiology) and ADVA (virology analysis) files (R24) surfaced several new anomalies (full HTML report). The workflow normalizes and compares four files:
xpt_data/FDA-CBER-2021-5683-0123168–0126026_125742_S1_M5_c4591001-A-D-adva.xptxpt_data/FDA-CBER-2021-5683-0282366–0285643_125742_S1_M5_c4591001-S-D-mb.xpteua_data/xpt_data/FDA-CBER-2021-5683-1495707–1496004_27034_S1_M5_c4591001-safety-fa-eff-A-adva.xpteua_data/xpt_data/FDA-CBER-2021-5683-1559614–1561992_27034_S1_M5_c4591001-safety-fa-eff-S-mb.xpt
Result mismatches.
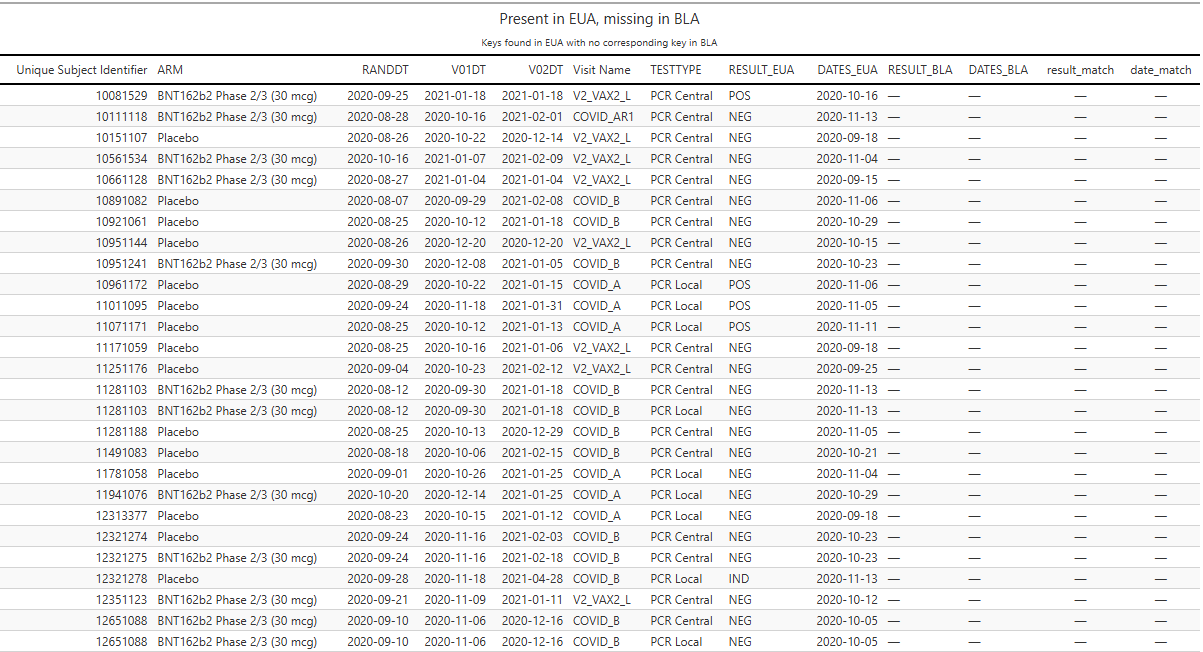
Example: participant 12312463 (BNT162b2 Phase 2/3, 30 µg) has a central-lab PCR on 2020-09-22 recorded as NEG in the EUA cut and POS in the BLA cut. The same flip occurs for 12312805 (BNT) and 12511022 (BNT).
Record disappearances.
Some tests present at EUA are simply absent at BLA.
Conclusion
Based on the analyses above (and the former), I find substantial, systematic inconsistencies between the EUA and BLA datasets - date shifts, result flips, record disappearances, and reclassifications. Taken together, these patterns are consistent with either the use of parallel datasets or extensive post-hoc edits. Without access to full audit trails and derivation programs, intent cannot be determined.
Such patterns would likely require actions at the data-management level (e.g., query resolutions, back-edits, re-derivations, or record re-keys). Determining responsibility demands a review of the electronic audit logs and change histories maintained by the data-management organization (ICON) and the sponsors. The presence of CRFs with embedded image inserts and wide-scale date edits is incompatible with GCP/ALCOA+ data-integrity principles and warrants independent verification.
The extent of involvement by other companies - such as Oracle, which supported data collection25 - should also be clarified. Particularly given that Oracle’s CEO, Larry Ellison, brags openly about being the one behind the allowance of these products to pregnant women26.
Jon Cohen - Fact check: No evidence supports Trump's claim that COVID-19 vaccine result was suppressed to sway election - https://www.science.org/content/article/fact-check-no-evidence-supports-trump-s-claim-covid-19-vaccine-result-was-suppressed
The New England Journal of Medicine - Fernando P. Polack et al. - Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine - https://www.nejm.org/doi/full/10.1056/nejmoa2034577
The New England Journal of Medicine - Stephen J. Thomas, M.D. et al. - Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months - https://www.nejm.org/doi/10.1056/NEJMoa2110345
ICANDECIDE.org - BREAKING: ICAN Releases More Than Half A Million Pages Of New Pfizer Vaccine EUA Data - https://icandecide.org/press-release/breaking-ican-releases-more-than-half-a-million-pages-of-new-pfizer-vaccine-eua-data/
BMJ 2022;378:o1731 - Josh A Guetzkow, Retsef Levi - Covid-19: Researchers face wait for patient level data from Pfizer and Moderna vaccine trials - https://www.bmj.com/content/378/bmj.o1731/rr-2
R-4.4.0 for Windows - https://cran.r-project.org/bin/windows/base
PHMPT.org - https://phmpt.org/multiple-file-downloads/
coviddocuments.com - https://coviddocuments.com/data-dictionary.html
R Script - download_eua.R - https://github.com/OpenVaet/pfizer_docs_R/blob/main/download_eua.R
R Script - extract_eua.R - https://github.com/OpenVaet/pfizer_docs_R/blob/main/extract_eua.R
R Script - verify_aes_dates.R - https://github.com/OpenVaet/pfizer_docs_R/blob/main/verify_aes_dates.R
R Script - eua_bla_aes_subjects.R - https://github.com/OpenVaet/pfizer_docs_R/blob/main/eua_bla_aes_subjects.R
R Script - compare_eua_bla_adsl_data.R - https://github.com/OpenVaet/pfizer_docs_R/blob/main/compare_eua_bla_adsl_data.R
X - Suggestion to the authors - https://x.com/canceledmouse/status/1715545558293590458
PHMPT.org - Analysis Data Reviewer Guide - https://phmpt.org/wp-content/uploads/2022/03/125742_S1_M5_c4591001-A-adrg.pdf
R Script - verify_testing_visit_dates.R - https://github.com/OpenVaet/pfizer_docs_R/blob/main/verify_testing_visit_dates.R
diginomica.com - Den Howlett - Oracle and COVID as told by Larry Ellison, chairman and CTO - https://diginomica.com/oracle-and-covid-told-larry-ellison-chairman-and-cto








Great work. Surely this is enough evidence now to stop administering the crap, and a thorough investigation.
The guy in the clip makes a joke that they allowed pregnant women in the study because not all women know when they are pregnant - so the researchers allegedly didn’t know it, either.
But how was it possible? My guess is that the participants in a study, right on the admission, are subjected to a number of tests (including blood or urine tests) with the purpose of assessing their general health condition and eligibility for the study.
In all medicine, pregnant women are considered at high risk and all procedures are fenced with enhanced safety requirements.
Now imagine one of the largest companies in the industry, working on a humanity-saving preparation, announcing full transparency like never before, and deliberately omitting the tests that could even hint at pregnancy.