Pfizer/BioNTech C4591001 Trial - Positive PCRs & Symptomatic Subjects
Looking for the positive PCR & Symptomatic subjects through Pfizer/BioNTech's data obfuscations - and uncovering some more major anomalies on the way...
We, once again, thanks Josh Guetzkow1 & Geoff Pain2 , for their precious assistance and key comments, going through this analysis.
Arkmedic (whom you can follow on Substack3 or on Telegram4), Jikkyleaks5, the DailyClout’s team 36 & Abstractor team7, Twitter’s “A Concerned Amyloidosis”8 and bio-statistician Christine Cotton9 have also provided key contributions in deepening our understanding of the Pfizer/BioNTech C4591001 Trial.
We suggest you to follow them, as without any doubt more is coming from these amazing researchers.
Introduction
As our readers are probably tired of reading, the EUA for the Pfizer/BioNTech “vaccines” was granted on the basis of an assumed efficacy of 95%, with a very restrictive criterion of “preventing a positive Sars-Cov-2 PCR, with symptoms, on subjects who hadn’t been confirmed positive at least 7 days post dose 2”.
(You can click on the pictures to expand them)
Getting these products to market was therefore requiring to :
Either have an actually “safe and effective” product.
Or to control the determination of the “Safe” and “Effective” parameters.
Following a famous Mouse tip that the subjects could very well have been “unblinded” at Pearl-River’s level, by running a simple PCR test on a subject’s sample post injection...


... we verified the symptomatic cases’ data noted page 41 of the FDA memorandum10.
Results
This article goes in detail on how to identify as accurately as possible, given the current sponsor’s data communicated through PHMPT lawsuits:
The symptomatic cases in the sponsors’ raw data.
The Positive PCRs.
The Positive PCRs with symptoms.
The “Suspected but non confirmed” Covid cases.
We highlight below a few more major anomalies (missing subjects from the analysis, subject in the efficacy group without documented symptoms, etc.).
Symptomatic Cases
A "symptomatic case" was defined by the protocol as a subject experiencing at least one of the “official covid-like” symptoms listed11:
• Chills
• Diarrhea
• Fever
• New loss of taste or smell
• New or increased cough
• New or increased muscle pain
• New or increased shortness of breath
• Sore throat
• Vomiting
To these we must add the “secondary endpoints” symptoms, detailed in the same page:
• Fatigue
• Headache
• Nasal congestion or runny nose
• Nausea.
These symptoms were collected in a SAS .XPT file, “S1_M5_c4591001 A D adsympt”12.
This file contains 328 439 rows with the variable 'PARCAT1' set to 'SIGNS AND SYMPTOMS OF DISEASE', on 9 025 subjects who reported such symptoms during the trial. Another key variable in this file is “AVISIT”, which keeps track of the occurrences by subjects of “suspected Covid” cases (with the variable set to “COVID_A”, “COVID_B”, etc.). In other words, a subject could have multiple covid visits according to certain criteria specified in the protocol. We are treating each of these distinct COVID visits as a separate "potential COVID case."
At cut-off date of November 14, 2020, symptoms had been registered on 1 872 occurrences (subject-visit(s) reporting symptoms) in the BNT162b2 group, 2 320 occurrences in the Placebo group, and 3 occurrences of unknown group, for a total of 4 195 occurrences. These were reported on 1758 BNT162b2 Subjects, 2196 Placebo subjects, and 3 subjects of unknown randomization group, for a total of 3957 subjects.
Complete list of “symptomatic” cases can be downloaded in this Google Spreadsheet.
Beware a subject may have more than one row of data (each Subject - Reporting date is unique).
Verifying the FDA statement on “suspected but unconfirmed Covid” was then requiring to identify accurately the symptomatic cases which had been confirmed.
Weekly Cumulative Symptomatic Cases by Treatment Arm
The following chart summarizes weekly accrual of symptomatic cases by treatment arms.
PCRs & Positive PCRs.
The SAS .XPT file “S1_M5_c4591001-S-D-mb” contains, among other Hepatitis or HIV tests, the raw results of the PCR tests performed on the subjects.
This file contains the data of 88 425 PCRs, on 43 645 subjects, at cut-off date of November 14, 2020.
Among these, 835 subjects tested positive on 1 037 total positive tests - including one phase 1 subject - which we excluded from further analytics.
We haven’t cared about “efficacy eligibility” here - but exclusively about who had a positive PCR registered or didn’t - and only about phase 3 subjects.
Complete list of the 835 “positive” subjects & their first positive PCR’s date can be downloaded in this Google Spreadsheet.
Complete list of PCR tests & their results can be downloaded in this Google Spreadsheet.
Positive PCRs with Symptoms
In order to be qualified as “positive PCR with symptoms”, the subjects had to accumulate the three conditions of:
Having at least one of the "covid-like" symptoms mentioned above13.
Having a positive for Sars-Cov-2 in a Cepheid RT-PCR assay for SARS-CoV-2 centralized at Pearl River - the main Pfizer laboratory (when the local laboratory results were conflicting with the central ones, only the central one was sustained)14.
That PCR confirmation had to happen within 3 to 5 days before or after the appearance of the symptoms, for the case to be considered “Positive with symptoms” (That precise time window is yet unclear: "within 4 days" appear to be specified in the FDA memorandum, with an "optimal 3 days after the onset of the symptoms" mentioned in the Protocol, while the .PDF data tells us it could go up to +/- 5 days).
Various .PDF files have been provided to “back” these figures. These are the following:
pd-production-030122/125742_S1_M5_5351_c4591001-fa-interim-lab-measurements.pdf
pd-production-070122/125742_S1_M5_5351_c4591001-interim-mth6-lab-measurements-sensitive.pdf
Unfortunately .PDFs are a doubtful source of information as “second hand” exports which already have been through the code of the study authors, and are requiring a lot of manual verification of the conversions to ensure the reliability of the analysis.
Break-down of these 835 subjects, depending on the symptoms reported 3, 4 & 5 days before or after a positive PCR, are summarized below:
Obviously, with the increase of days between symptoms & positive PCRs, the total of “cases accrued” increases - and we have 371 to 376 subjects with a positive PCR & concomitant symptom(s).
Complete list of “positive with symptoms” subjects concerned & their first positive PCR data and symptoms details can be downloaded on this Google Spreadsheet.
Weekly Cumulative PCR-Confirmed Symptomatic Cases, by Treatment Arm
The following chart summarizes weekly accrual of positive PCR & symptomatic cases by treatment arms.
This chart is initiated on the same week 31 than the symptoms.
.PDF to Symptomatic Positive PCRs - Subjects cross-check
As a further cross-check of the relevance of our analysis, we verified our results against the 1180 positive cases listed in the .PDF files:
pd-production-030122/125742_S1_M5_5351_c4591001-fa-interim-lab-measurements.pdf
pd-production-070122/125742_S1_M5_5351_c4591001-interim-mth6-lab-measurements-sensitive.pdf
These files contain data on 363 “phase 3” subjects (55 BNT162b2 & 308 Placebo) who had a positive PCR & at least one symptom up to November 14.
Optimal coverage of the cases listed in the .PDF is obtained at +/- 5 days, including “secondary end-point” symptoms, which generates 61 BNT162, 314 Placebos & 1 Unknown randomization group, for a total of 376 subjects.
Subjects documented in the .PDF files while missing in our analysis.
2 Placebo subjects featured in the .PDF (10111148, 10911203) aren’t found in our analysis, even at 15 days, while they are present in the .PDF files:
10911203 made it to the efficacy population (the most important 170 confirmed cases used as basis for the 95% efficacy) - this despite an "unknown" Central lab test result15 (Which is the parameter not satisfied for him to be featured here)…
10111148 only appears in the “Month 6 Lab Measurements” (not on the November Interim Analysis). He does not have recorded concomitant symptoms. The .PDF itself places its symptoms start to October 6, a month before.
The two subjects are quite specific, as they are declared positive while having a negative PCR & a positive “repeat central swab 1” (R1) in the 6 months lab measurements file16.
Subjects found via our analysis while missing in the .PDF files.
15 subjects we find as “positive with symptoms” aren’t listed in the .PDF prior to cut-off date (1 unknown, 8 Placebo, 6 BNT162).
Subject 10571312, BNT162, PCR positive on November 4, reporting Cough on November 9, 5 days later (Excluded for not receiving two doses).
Subject 10931111, BNT162, PCR positive on September 28, reporting Rhinorrhoea (runny nose) on the same day (Appears in the Adverse events for Dizziness17, but no documented exclusion).
It's worth noting that 10931111 doesn't have any "official" symptom, only secondary ones - but there are subjects who were included in the .PDF of COVID cases even though they only have secondary symptoms - for example 11471037, 11781118, and others).
Subject 10941132, BNT162, PCR positive on October 7, reporting fever, loss of taste or smell & cough on the same day (Appears in the Adverse events for Hyperhidrosis/ sweat, but no documented exclusion).
Subject 11331263, BNT162, PCR positive on August 26, reporting cough & sore throat on the same day (Appears in the Adverse events for Malaise, Cough, Fatigue, Injection site pain, Musculoskeletal discomfort, Headache, but no documented exclusion).
Subject 12314205, BNT162, PCR positive on September 14, reporting loss of taste or smell the same day (Discontinued for “Clinical diagnosis of Covid 19”18, Excluded for not receiving two doses).
Subject 12481005, BNT162, PCR positive on August 26, reporting cough & shortness of breath on the same day (Appears in the Adverse events for Photophobia/ Light Sensitivity, Ear infection, Vertigo, Excluded for “other important protocol deviations on or prior to 7 days after Dose 2”).
Subject 10971102, Placebo, PCR positive on October 12, reporting cough on the same day (No documented exclusion or adverse event reported prior to cut-off)
Subject 11411069, Placebo, PCR positive on October 25, reporting loss of taste or smell the same day (No documented exclusion or adverse event reported prior to cut-off)
Subject 11611008, Placebo, PCR positive on November 10, reporting chills, headache, nausea, loss of taste or smell, cough & Rhinorrhoea the same day (Sensitive deviation noted for “Participant met exclusion criterion #22 (investigator site staff or Pfizer employees directly involved in the conduct of the study, site staff otherwise supervised by the investigator, and their respective family members)”, Excluded for not receiving two doses)
Subject 11681221, Placebo, PCR positive on October 29, reporting diarrhea, fever, headache, loss of taste or smell, cough, muscle pain, shortness of breath on November 3, 5 days later (Discontinued on December 21, post cut-off, for choosing not to participate to the study anymore)
Subject 12312097, Placebo, PCR positive on October 28, reporting chills, headache, muscle pain, shortness of breath the same day (No documented exclusion or adverse event reported prior to cut-off)
Subject 12313879, Placebo, PCR positive on September 13, reporting cough 2 days earlier, on September 11 (Appears in the Adverse events for Injection site pain, Exposure during pregnancy, headache. Yes, subject is a female. No exclusion is documented)
Subject 12315608, Placebo, PCR positive on August 31, reporting loss of taste or smell and sore throat 4 days later, on September 4 (Has a deviation “Participant failed to meet inclusion criterion #03 (Healthy participants who are determined by medical history, physical examination and clinical judgement of the investigator to be eligible for inclusion in the study)”, a discontinuation or “Previous clinical or microbiological diagnosis of COVID19”, and an exclusion for “Did not receive 2 vaccinations” - take your pick…)
Subject 12351060, Placebo, PCR positive on October 23, reporting muscle pain a day before, on October 22 (Appears in the adverse effects in January 2021 but not before data cut-off)
Subject 44441622, randomization group unknown, isn’t lucky enough to appear in any .PDF. The FDA-CBER-2021-5683-0593744-0593769-125742_S1_M5_bnt162-01-S-Supp-D-suppds.xpt file tells us he very likely has been screened on September 23, went far enough to be positive on a PCR on the same day, and to report Chills, fever, cough, but was considered as a screening failure19.
Some subjects in this above list (11331263, 12481005 - both BNT162b2 recipients) would have qualified as “PCR positive with symptoms” on the day of their first dose. We verified if this parameter would have been a valid cause for them not to be documented, and several documented cases are fitting that scenario - but as rightfully observed by Josh Guetzkow, they are also systematically tested again, a few days after their first dose:
subject 10051341 (Placebo) - Positive PCR on 2020-10-21, dose 1 on 2020-10-21, first symptoms on 2020-10-23. Documented through another Positive PCR on October 24 in the lab measurement documents.
subject 10391021 (Placebo) - Positive PCR on 2020-08-25, dose 1 on 2020-08-25, first symptoms on 2020-08-28. Documented through another Positive PCR on August 28 in the lab measurement documents.
subject 12262267 (Placebo) - Positive PCR on 2020-10-17, dose 1 on 2020-10-17, first symptoms on 2020-10-20. Documented through another Positive PCR on October 20 in the lab measurement documents.
subject 12313225 (BNT162b2) - Positive PCR on 2020-08-22, dose 1 on 2020-08-22, first symptoms on 2020-08-25. Documented through another Positive PCR on August 25 in the lab measurement documents.
subject 12313395 (Placebo) - Positive PCR on 2020-08-23, dose 1 on 2020-08-23, first symptoms on 2020-08-24. Documented through another Positive PCR on August 24 in the lab measurement documents.
subject 12314314 (BNT162b2) - Positive PCR on 2020-08-26, dose 1 on 2020-08-26, first symptoms on 2020-08-29. Documented through another Positive PCR on August 29 in the lab measurement documents.
subject 12314492 (Placebo) - Positive PCR on 2020-08-27, dose 1 on 2020-08-27, first symptoms on 2020-08-31. Documented through another Positive PCR on August 31 in the lab measurement documents.
Note 4 of these 7 specific subjects are coming from Site 1231, led by Fernando Polack, lead author of the Phase 3 study and Coalition for Epidemic Preparedness Innovations (CEPI) member.
Identifying the “suspected but unconfirmed” Covid cases.
At this stage we have at our disposal a list of “suspected covid” visits of subjects reporting symptoms, and we know which “suspected covid” resulted in confirmed cases (minus, of course, the few they have forgotten and which we detailed above).
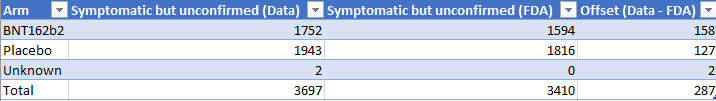
377 cases of suspected Sars-Cov-2 have been confirmed on the 376 subjects. 3 818 cases (on a total of 4195) haven’t been suspected but unconfirmed; 1811 BNT & 2005 placebo.
Assuming that our data-based settings above are correct (+/- 5 days, official & secondary symptoms), the offsets between the FDA & data figures are the following:
We verified several hypothesis to try to explain this 408 subjects offset (217 BNT, 189 Placebo & 2 unknown).
Excluding the 195 phase 1 active subjects - whose fevers are hidden in another XPT file, “S1_M5_c4591001-S-D-ce”, has no effect on that offset.
Excluding subjects with only one symptom among the targeted ones would exclude 638 BNT162b2, 714 Placebos and 1 Unknown, and would bring the total far below the FDA’s one.
We verified if no children under 16 were included in the data; that’s not the case.
We verified if excluded subjects prior to November 14, 2020 could explain the discrepancy. By lack of accurate XPT data on exclusions all we have located to date is a November 24, 2020 .PDF20. This would exclude 333 BNT162b2 & 379 Placebo, bringing the totals yet again below FDA figures.
We verified if the cut-off date could have been earlier for these figures. This yields no relevant result.
We verified how including only “Official symptoms” in that last listing would affect the count. (+/- 5 days, official symptoms only), the offsets between the FDA & data figures are the following:
This brings us to the closest total we found to these FDA figures.
The complete list of these “suspected but unconfirmed cases” - with or without official symptoms - can be downloaded on this Google Spreadsheet.
Being out of ideas to explain this offset without altering the sense of the memorandum text, and being quite accustomed to Pfizer’s synthesis not matching the raw data21, let’s settle on this first 1811 BNT162b2 & 2005 Placebo “suspected but not confirmed” which we can actually back with data, until someone has a bright idea we can test in the comments.
This article re-uses code & data already detailed in the “Methodology Details” of our article “Pfizer/BioNTech Trial - A failed yet in depth attempt to reproduce the NEJM & FDA efficacy figures”22.
The code itself used to parse the various extracts & generated the exports featured here is accessible on GitHub.
This report is part of an ongoing work in progress. As a quite complex task, going through a large clinical trial on which a lot of efforts have been deployed so the public wouldn’t have a clear view, none of its findings should be used without an appropriate in-depth cross-check of the results. We’re always open to discuss our results and to rectify swiftly errors we may have made - and we will withdraw this warning as soon as the code will have been double-checked and will have gone through in depth review on our end.
fda.gov/media/144416/download, page 14.
fda.gov/media/144416/download, page 14.
phmpt.org/wp-content/uploads/2022/03/125742_S1_M5_5351_c4591001-fa-interim-lab-measurements-sensitive.pdf - 16.2.8.2 Listing of Subjects With First COVID-19 Occurrence From 7 Days After Dose 2 and Without Evidence of Infection Prior to 7 Days After Dose 2 – Evaluable Efficacy (7 Days) Population
fda.gov/media/144416/download, page 18.










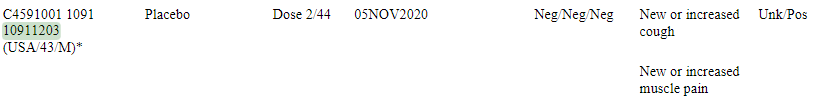






The trials were completely faked and incomplete. That is because this substance is not a vaccine or even a fake vaccine. It is a prototype countermeasure that was contracted by the DOD to be manufactured and distributed by big pharma and green-lighted by the FDA without questions.
Since it was an undefined (as far as exact purpose goes) countermeasure instigated under the EUA, no trials or testing was necessary before it hit the public. According to the DOD/big pharma contracts, this mRNA substance IS NOT a pharmaceutical. Therefore, it did not require ANY RCT trials.
It was promoted as a safe and effective vaccine but big pharma was acting under contractual orders of the DOD and HHS. It was a lie from day one.
I don't have the techie skills to plod through the stats, and I greatly appreciate that you all are able and willing. But I can contribute something else here.
What caught my attention in the article was this, shown in the screen capture of Pfizer's memo to the FDA asking for EUA (online date 11/dec/2020):
“It is possible that the imbalance in suspected COVID-19 cases [409 pfizered vs. 287 placebo] occurring in the 7 days post vaccination **represents vaccine reactogenicity** with **symptoms that overlap with those of COVID-19.** Overall though, these data do not raise a concern that protocol-specified reporting of suspected but unconfirmed COVID-19 cases could have masked clinically significant adverse events **that would not have otherwise been detected.**” (star highlights added)
First highlight:
Looking up "reactogenicity" revealed a lot of studies over the past 2 years were trying to verify the universal public reassurance that if you have serious AEs from the jab, "it means you're getting a good immune response."
I random-checked some peer-reviewed articles... from Germany, South Korea, Saudi Arabia, and China.
None of them could find a correlation between reactogenicity and immunogenicity. Not even the Chinese, who lied in their abstract but admitted the truth in their results.
In fact, the Germans described this idea as "a common belief" with "limited data" [read: none] to support it. And the Koreans flatly said that "reactogenicity… cannot be assumed to correlate with immunogenicity.”
So it's a myth. Yet the CDC website, updated just this month, repeats that AE reactions from the mRNA jab "are normal signs the body is building immunity."
Second/third highlights:
Remember the alarmingly long duration of the "clinically significant" AEs which we now know among the jabbed, and put it together with this neat identification of the AEs with what came to be called "long Covid" symptoms. We see that Pfizer knew about it from the start. And so did the FDA.
Presumably that includes the AEs causing "sudden death syndrome" - which we now know clustered within 14 days of the jab and were thus written off as "unvaxxed" deaths, or even "Covid" deaths. These too had "otherwise been detected" already by Pfizer and were expected to occur during the global roll-out.
The question is: Does knowing any of this, after the fact, help the cause of justice?